Solitary Pulmonary Nodule: Overview, Types of Benign Pulmonary Tumors, Etiology
Solitary Pulmonary Nodule: Overview, Types of Benign Pulmonary Tumors, Etiology
A solitary pulmonary nodule is defined as a discrete, well-marginated, rounded opacity less than or equal to 3 cm in diameter that is completely surrounded by lung parenchyma, does not touch the hilum or mediastinum, and is not associated with adenopathy, atelectasis, or pleural effusion. Lesions larger than 3 cm are considered masses and are treated as malignancies until proven otherwise. (See the images below.)
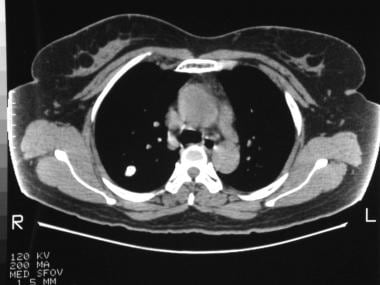 Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.
Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.
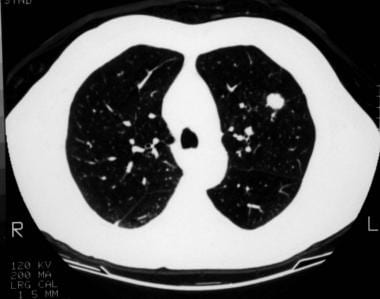 A 1.5-cm coin lesion in the left upper lobe in a patient with prior colonic carcinoma. Transthoracic needle biopsy findings confirmed this to be a metastatic deposit.
A 1.5-cm coin lesion in the left upper lobe in a patient with prior colonic carcinoma. Transthoracic needle biopsy findings confirmed this to be a metastatic deposit.
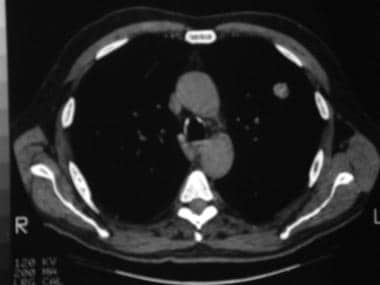 Mediastinal windows of the patient in the previous image
Mediastinal windows of the patient in the previous image
See The Solitary Pulmonary Nodule: Is It Lung Cancer?, a Critical Images slideshow, for more information on benign and malignant etiologies of solitary pulmonary nodules.
Patients with solitary pulmonary nodules are usually asymptomatic. However, solitary pulmonary nodules can pose a challenge to clinicians and patients. Whether detected serendipitously or during a routine investigation, a nodule on a chest radiograph raises the following questions:
Most solitary pulmonary nodules are benign. However, they may represent an early stage of lung cancer. Lung cancer is the leading cause of cancer death in the United States, accounting for more deaths annually than breast, colon, and prostate cancers combined. Lung cancer survival rates remain dismally low at 14% at 5 years.
Patients with early lung cancer, when the primary tumor is less than 3 cm in diameter without evidence of lymph node involvement or distant metastasis (stage 1A), have a 5-year survival rate of 70-80%. Therefore, prompt diagnosis and management of early lung cancer manifesting as a solitary pulmonary nodule is the the best chance for cure.
Benign lung tumors are a heterogenous group of neoplastic lesions originating from pulmonary structures. These tumors include bronchial adenomas, hamartomas, and a group of uncommon neoplasms (eg, chondromas, fibromas, lipomas, leiomyomas, hemangiomas, teratomas, pseudolymphomas, endometrioma, and bronchial glomus tumors).
Although benign lung tumors do not pose a significant health problem, complications can result if an obstructive lesion predisposes the patient to pneumonia, atelectasis, and hemoptysis.
Determination of whether a lung nodule is benign or malignant based solely on its anatomical location is an incorrect practice. Anatomical location has no predictability on the malignant potential of a tumor. Benign lung tumors can occur in the periphery of the lung, but they can also occur as endobronchial lesions within the tracheobronchial tree.
Characteristics
Neoplastic lesions are characterized by the autonomous proliferation of cells without a response to the normal control mechanisms governing cell growth. An additional characteristic of benign tumors is extension without local tissue invasion or spread to other sites.
Hamartomas consist of haphazardly organized mature cells and tissues. Hamartomas are composed mostly of masses of hyaline cartilage with a myxoid connective tissue, adipose cells, smooth muscle cells, and clefts lined with respiratory epithelium. (See the image below.)
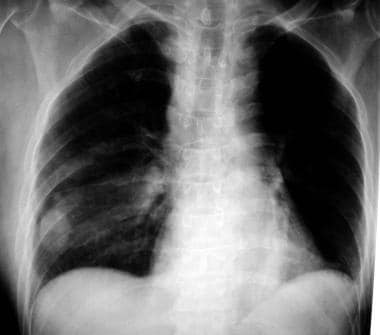 A right lower lobe solitary pulmonary nodule that was later identified as a hamartoma.
A right lower lobe solitary pulmonary nodule that was later identified as a hamartoma.
Classification
Benign lung tumors can be classified pathologically, but a clinically useful classification would combine location (ie, endobronchial or parenchymal) and information about whether the lesions are single or multiple. Benign lung tumors can also be classified by their presumed origin. Those classifications include the following:
Adenomas and hamartomas constitute the largest group of benign lung tumors and, thus, deserve detailed descriptions.
Generally, a pulmonary nodule must reach 1 cm in diameter before it can be identified on a chest radiograph. For a malignant nodule to reach this size, approximately 30 doublings would have occurred. The average doubling time for a malignant tumor is 120 days (range 7-590 d). A lesion at this growth rate may be present for 10 years before discovery.
For patient education information, see the Cancer Center, as well as Bronchoscopy and Bronchial Adenoma.
A solitary pulmonary nodule may be secondary to a wide differential of causes. However, more than 95% are malignancies (most likely primary), granulomas (most likely infectious), or benign tumors (most likely hamartomas).
The cause and pathogenesis of benign lung tumors are poorly understood. The nomenclature of benign lung tumors is based on histologic findings. Some of these tumors have benign features, while others are hamartomas.
Hamartomas (chondroadenomas) are the most common type of benign lung tumor. They occur primarily in adults, although they do occasionally arise in children. Hamartomas are peripherally located. Grossly, they have a firm, marblelike consistency. Histologically, hamartomas generally consist of epithelial tissue and other tissues, such as fat and cartilage. Hamartomas can be easily enucleated, but wedge resection is also appropriate.
Bronchial adenomas make up 50% of all benign pulmonary tumors. The term bronchial adenoma should be discouraged because, when used loosely, it includes carcinoid tumors, adenocystic carcinomas, and mucoepidermoid carcinomas, which, in fact, are low-grade malignant tumors.
Mucous gland adenomas are true benign bronchial adenomas. Mucous gland adenomas, which are also called bronchial cystadenomas, arise in the main or local bronchi. Histologically, they consist of columnar cell–lined cystic spaces with a papillary appearance.
Multiple laryngeal papillomatosis is a viral disease of the upper airway that primarily affects children. This disorder has malignant potential and may later spread to the tracheobronchial tree.
Solitary papillomas usually are less than 1.5 cm in diameter. They usually are lobar or segmental in location and are histologically similar to viral papillomatosis.
Inflammatory papilloma is a solitary polypoid mass of granulation tissue that is associated with an underlying pulmonary inflammatory condition.
Granular cell myoblastomas are of neural cell origin. A granular cell myoblastoma contains polygonal or spindle cells with granular cytoplasm. Granular cell myoblastomas tend to be multiple in 10% of cases and are more common in men aged 30-50 years.
Other parenchymal tumors occasionally occurring in the endobronchial tree (eg, leiomyoma, lipoma) almost exclusively are found at an endobronchial location.
Sclerosing hemangioma is an uncommon tumor derived from the epithelial cells of pneumocytes (terminal bronchiolar cells). This tumor consists of several elements, including solid cellular areas, papillary structures, sclerotic regions, and blood-filled spaces. Sclerosing hemangiomas are most commonly found in middle-aged women. Chest radiography demonstrates a well-defined nodule that is less than 3 cm.
Other mesenchymal tumors include lipoma, leiomyoma, neural tumors, fibroma, benign clear-cell tumor, teratoma, plasma cell granuloma, fibrous histiocytoma, xanthoma, pulmonary hyalinizing granuloma, pulmonary endometrioma, and pseudolymphoma.
Many benign lung tumors occasionally have multiple origins. Among these are hamartomas, hyalinizing granulomas, leiomyomas, and sclerosing hemangiomas.
The Carney triad is a syndrome of gastric epithelioid leiomyosarcoma, pulmonary chondromas, and extra-adrenal paragangliomas. The Carney triad mainly affects women.
Pulmonary tumorlets are minute collections of neuroendocrine cells scattered throughout the lung. Pulmonary tumorlets predominantly affect older women.
Clinically significant intrapulmonary chemodectomas apparently are paragangliomas. They behave in a benign fashion.
Bearing in mind that the major distinction that must be made is between neoplastic and inflammatory lesions, solitary pulmonary nodules have several causes:
Neoplastic (malignant or benign) tumors can be caused by the following:
Inflammatory (infectious) nodules can result from the following:
Inflammatory (noninfectious) nodules can be caused by the following:
Congenital nodules can be produced by the following:
Other causes of pulmonary nodules include the following:
Solitary pulmonary nodules are one of the most common thoracic radiographic abnormalities. Approximately 150,000 cases are detected each year as an incidental finding, either on chest radiographs or on thoracic computed tomography (CT) scans.In lung cancer screening studies that enrolled people at high risk for lung cancer, the prevalence of solitary pulmonary nodules ranged from 8-51%.
Approximately 40-50% of solitary pulmonary nodules are malignant. Gould et al reported after a review of the literature that most of these are adenocarcinoma (47%), followed by squamous cell carcinoma (22%). Small cell lung cancer makes up only 4% of malignant solitary pulmonary nodules.
Reported series suggest that benign lung tumors affect men more frequently than women (adenoma and hamartoma).
Risk of malignancy increases with age. For individuals younger than 39 years, the risk is 3%. The risk increases to 15% for individuals aged 40-49 years, to 43% for persons aged 50-59 years, and to more than 50% for persons older than 60 years.
Surgical resection is curative for most benign lung tumors. The 5- and 10-year survival rates following surgical resection of typical carcinoid tumors of the lung are 95% and 90%, respectively. The 5- and 10-year survival rates for patients with atypical carcinoids are 40-70% and 18-50%, respectively.
In one study, complete bronchoscopic resection for endobronchial carcinoid tumors at 1 and 10 years provided disease-free states at rates of 100% and 94%, respectively. (See the images below.)
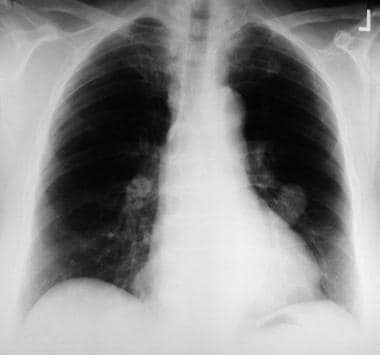 This left lower lobe carcinoid tumor was quite bloody after a percutaneous needle biopsy was performed.
This left lower lobe carcinoid tumor was quite bloody after a percutaneous needle biopsy was performed.
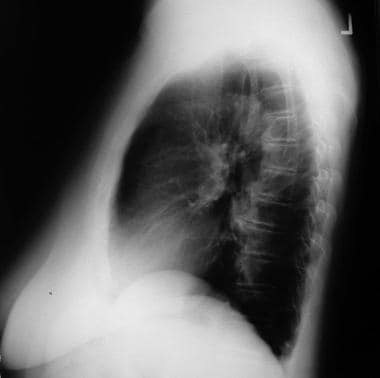 Lateral radiograph of the patient in the previous image.
Lateral radiograph of the patient in the previous image.
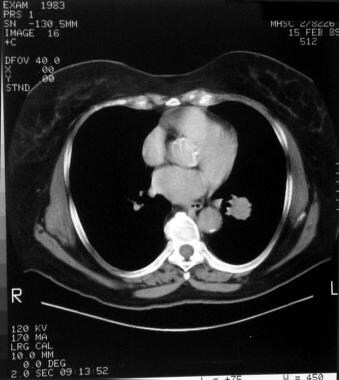 Computed tomography (CT) scan of a patient with a left lower lobe carcinoid tumor shows a well-circumscribed lesion.
Computed tomography (CT) scan of a patient with a left lower lobe carcinoid tumor shows a well-circumscribed lesion.
Although most solitary pulmonary nodules are benign, they may represent an early stage of lung cancer. While lung cancer survival rates remain dismally low at 14% at 5 years, diagnosis of early lung cancer (ie, when the primary tumor has a diameter smaller than 3 cm with no lymph node involvement and no distant metastasis [stage 1A]) can be associated with a 5-year survival rate of 70-80%. Accordingly, the best chance for cure of early lung cancer manifesting as a solitary pulmonary nodule is prompt diagnosis and management.
Possible complications due to benign lung tumors include pneumonia, atelectasis, hemoptysis, hyperinflation, and malignancy.
Most patients with solitary pulmonary nodules are asymptomatic; the nodules are typically detected as an incidental finding. Approximately 20-30% of all bronchogenic carcinomas appear as solitary pulmonary nodules on initial radiographs. The following features are important when assessing whether the nodule is benign or malignant:
The diagnostic approach is basically the same for these tumors, with clinical presentations depending on the location of the tumor (eg, trachea, other airways, parenchyma). Hemoptysis, lung mass on chest radiograph, and unresolved infiltrates may be present. Symptoms may include the following:
Most patients are asymptomatic from their solitary pulmonary nodules. If a patient is asymptomatic, the tumor is typically identified serendipitously.
The following conditions should be considered in the differential diagnosis of a solitary pulmonary nodule:
Because a malignancy may be curable when present as a solitary pulmonary nodule, great care should be taken in evaluating such lesions. A comprehensive assessment generally includes history, physical examination, evaluation of previous chest radiographs, incorporation of other imaging studies (eg, CT scanning, positron-emission tomography [PET] scanning, single-photon emission CT [SPECT] scanning), and invasive diagnostic procedures.
Because determining the pretest probability of malignancy is essential in guiding the management of solitary pulmonary nodules, estimating the probability of benignity using a validated quantitative model might be an effective strategy. Bayesian analysis combines the radiologic features of a nodule and the clinical findings of an individual patient to estimate the probability of malignancy.
The radiographic features of solitary pulmonary nodules included in the Bayes analysis are size, edge, contour, cavity-wall thickness, and growth rate; the clinical parameters are smoking, age, previous malignancy, and hemoptysis. (These features and parameters have been derived from previously published series.)
The calculation of likelihood ratios and their use may result in fewer false-negative and false-positive results.
Because the evidence is not definitive for many of the management guidelines, clinicians should discuss with patients the risks and benefits of alternative management options and should elicit patient preferences. The probability of malignancy only provides an estimate based on previously published studies and may not be generalized to an individual patient. Therefore, patient preferences and clinician experience are important in planning further management strategies.
Laboratory studies have a limited role in the workup of solitary pulmonary nodules. Anemia or an elevated sedimentation rate may indicate an underlying neoplastic or infectious process, while elevated levels of liver enzymes, alkaline phosphatase, or serum calcium may indicate metastases from a solitary bronchogenic carcinoma or from a nonpulmonary malignancy.
Patients who have histoplasmosis or coccidioidomycosis may have high levels of immunoglobulin G and immunoglobulin M antibodies specific to these fungi.
A preoperative complete blood cell count should be performed on all patients who undergo tissue sampling. The cell count also helps to determine the general health status of the patient and in the diagnosis of complications such as pneumonia and anemia.
Coagulation (PT and INR, PTT) studies are recommended before the patient undergoes any invasive procedures. The adequacy of the platelet function should be determined only in selected patients.
Electrolytes and renal and liver function tests help to evaluate the presence of an abnormality that may indicate the need for either intervention or further workup before an invasive procedure is performed.
A tuberculin skin test and sputum cytologic and microbiological studies should also be performed in selected patients.
A patient with a carcinoid tumor, with or without carcinoid syndrome, may exhibit a high level of serotonin and 5-hydroxyindoleacetic acid (5-HIAA).
Arterial blood gas and pulmonary function tests (PFTs) are indicated in patients presenting with shortness of breath and are indicated before invasive procedures or thoracotomy. The presence of hypoxia and hypercarbia generally suggests poor tolerability for resective surgery. PFTs are useful tests when determining patients' suitability for lung resection. Patients must have satisfactory parameters as measured by forced expiratory volume in 1 second (FEV1) and diffusion capacity of lung for carbon monoxide (DLCO).
Because solitary pulmonary nodules are typically first detected on chest radiographs, the initial distinction made is whether the nodule is pulmonary or extrapulmonary in nature. Findings from lateral chest radiography, fluoroscopy, or CT scanning may help to confirm the location of the nodule. Although nodules of 5 mm in diameter are occasionally visualized on chest radiographs, solitary pulmonary nodules are quite often 8-10 mm in diameter.
Chest radiographs can provide information regarding size, shape, cavitation, growth rate, and calcification pattern. All of these radiologic features can help in determining whether the lesion is benign or malignant. However, none of these features is entirely specific for lung carcinoma.
CT scanning of the chest has many advantages over plain chest radiography.The advantages include better resolution of nodules and detection of nodules as small as 3-4 mm. CT scan images also help to better characterize the morphologic features of various lesions. Multiple nodules and regions that are difficult to assess on chest radiographs are better visualized on CT scan images. (See the images below.)
 A left upper lobe nodule with central lucency and poorly circumscribed margins was diagnosed as actinomycosis based on needle biopsy findings.
A left upper lobe nodule with central lucency and poorly circumscribed margins was diagnosed as actinomycosis based on needle biopsy findings.
 Computed tomography (CT) scan of the patient in the previous image. After needle biopsy, the presence of classic sulfur granules confirmed a diagnosis of actinomycosis.
Computed tomography (CT) scan of the patient in the previous image. After needle biopsy, the presence of classic sulfur granules confirmed a diagnosis of actinomycosis.
 A left upper lobe solitary pulmonary nodule. The differential diagnosis in such cases is large, but computed tomography (CT) scan findings help to narrow the differentials and establish the diagnosis.
A left upper lobe solitary pulmonary nodule. The differential diagnosis in such cases is large, but computed tomography (CT) scan findings help to narrow the differentials and establish the diagnosis.
CT densitometry measures the attenuation coefficients of a lesion and aids detection of occult calcification. Attenuation coefficients are expressed in Hounsfield units (HU); a value of more than 185 HU has been suggested as a cutoff for benign lesions. However, prospective studies have indicated low sensitivity and specificity for CT densitometry measurements. Thus, these measurements are no longer routinely employed.
With regard to dynamic contrast enhancement, a greater degree of contrast enhancement on repeated measurements of attenuation indicates that the nodule is malignant. Enhancement of greater than 20 HU is associated with malignancy, whereas less than 15 HU suggests a benign lesion. A multicenter study using a cutoff value of 15 HU found a sensitivity and specificity of 98% and 58%, respectively.Active granulomas or other infectious lesions may also enhance, limiting the application of this technique. However, a failure to enhance by more than 15-20 HU has a greater than a 95% predictive value for benignity.
Several radiologic characteristics found on CT scanning and radiography (although CT scanning is superior) may help to establish the diagnosis or suggest whether a lesion is benign or malignant. These include the following:
Although a well-defined nodule of smaller size that is clearly visible on chest radiographs may be calcified and benign, small lesions may very well be early stage bronchogenic carcinoma. A lesion greater than 4 cm in diameter is very likely a bronchogenic carcinoma, although exceptions include lung abscess, Wegener's granulomatosis, lymphoma, round pneumonia, rounded atelectasis, and hydatid cyst.
Midthun et al reported that the likelihood of malignancy was 50% for nodules greater than 20 mm and 18% for those 8-20 mm in diameter. With lesions smaller than 8 mm, a sharp decline is noted, with nodules of 4-7 mm having a likelihood of malignancy of only 0.9% and those less that 3 mm, only 0.2%.
Serial chest radiographs facilitate estimation of the growth rate of a nodule. Growth rate refers to the doubling time of a nodule, which is a doubling of the nodule volume. Because a nodule on a chest radiograph is seen as a 2-dimensional (2-D) circle rather than a 3-D sphere, an increase in diameter of 26% corresponds to a doubling of nodule volume.
Bronchogenic carcinoma generally doubles in 1-18 months (average 4-8 mo). Although a doubling time of less than 1 month or longer than 18 months makes bronchogenic carcinoma unlikely, it does not exclude the diagnosis completely. Important exceptions are bronchoalveolar carcinoma, which may require more than 2 years to double in size, and metastases from specific tumors (eg, osteosarcoma) that grow rapidly.
In general, doubling times of less than 1 month suggest infections; doubling times of more than 18 months suggest benign processes such as granuloma, hamartoma, bronchial carcinoid, and rounded atelectasis. If a nodule remains the same size for 2 years, it is very likely benign. However, further follow-up monitoring may be indicated.
In one retrospective series, a volume doubling time of less than 400 days at 3 months and 1 year follow-up was strongly predictive of malignancy.
Chest radiographs may demonstrate calcification, which often indicates that the lesion is benign. (CT scanning is the most sensitive technique for detection of calcification.) The 5 patterns of calcification usually observed in benign lesions are diffuse, central, laminar, concentric, and popcorn. A stippled or eccentric pattern is associated with malignancy. (See the images below.)
 Right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
Right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
 Close-up view of a right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
Close-up view of a right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
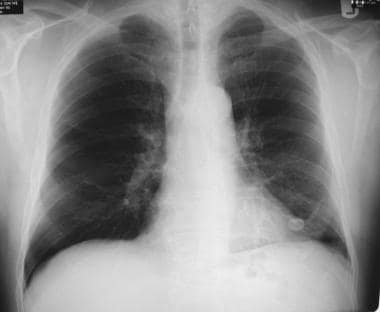 A popcorn calcification in the left lung nodule indicates a benign lesion or hamartoma. No further tests or observations were needed for this patient.
A popcorn calcification in the left lung nodule indicates a benign lesion or hamartoma. No further tests or observations were needed for this patient.
 Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.
Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.
A very irregular edge or corona radiata (numerous strands radiating into the surrounding lung) may indicate a bronchogenic carcinoma. Whereas lobulation and notching may indicate bronchogenic carcinoma, a well-defined, smooth, nonlobulated edge may indicate a benign lesion or metastasis.
Cavitation with a thin, smooth wall may indicate lung abscess or a benign lesion, whereas thick-walled cavitations imply an underlying malignant neoplasm. (See the images below.)
 Left upper lobe cavitating solitary nodule eventually identified as active pulmonary tuberculosis from percutaneous needle biopsy findings.
Left upper lobe cavitating solitary nodule eventually identified as active pulmonary tuberculosis from percutaneous needle biopsy findings.
 Cavitating right lower lobe nodule later confirmed to be primary pulmonary lymphoma. Calcium deposits may also be present in the lesion.
Cavitating right lower lobe nodule later confirmed to be primary pulmonary lymphoma. Calcium deposits may also be present in the lesion.
The CT-scan halo sign (ie, ground-glass attenuation surrounding a nodule on CT scan image) most commonly indicates infection with an invasive Aspergillus species. Other, less common possibilities include TB, cytomegalovirus infection, and herpes simplex infections.
Several characteristics within the nodule itself can indicate a specific cause. For example, demonstration of fat within the lesion is specific for a hamartoma, a benign lesion. (See the images below.)
 Left upper lobe 1.5-cm nodule shows negative computed tomography (CT) scan numbers, suggesting fat in the lesion consistent with hamartoma.
Left upper lobe 1.5-cm nodule shows negative computed tomography (CT) scan numbers, suggesting fat in the lesion consistent with hamartoma.
 The parenchymal lesion in this computed tomography (CT) scan demonstrates low attenuation within the lesion, indicating the presence of fat. Fat density is observed only in hamartoma and lipoid pneumonia. The likely diagnosis is hamartoma
The parenchymal lesion in this computed tomography (CT) scan demonstrates low attenuation within the lesion, indicating the presence of fat. Fat density is observed only in hamartoma and lipoid pneumonia. The likely diagnosis is hamartoma
Ground-glass opacities may represent a benign lesion, such as atypical adenomatous hyperplasia, or malignancy, such as bronchoalveolar carcinoma (adenocarcinoma in situ).Importantly, malignant ground-glass opacities often grow slower and may require longer follow-up.
Subsolid nodules (nodules with both a solid and a ground-glass component) are frequently peripheral adenocarcinomas of the lung. Studies have demonstrated excellent correlation between the Noguchi classification of adenocarcinomas and CT-scan findings. Specifically, atypical alveolar hyperplasia typically manifests as pure ground-glass lesions of less than 5 cm; bronchoalveolar carcinoma is usually greater than 5 cm. Lesions with a mixed solid component and ground glass correlate with adenocarcinoma, mixed subtype.
The presence of air bronchograms within the solitary pulmonary nodule makes bronchogenic carcinoma or metastasis unlikely, although they may be observed with bronchoalveolar carcinoma or lymphoma. Invasion of the adjacent bone by the nodule is pathognomic of bronchogenic carcinoma.
Nodules that are attached to pleura, vessels, or fissures are likely to be benign.
Whether positron-emission tomography (PET) scanning will be useful in a patient’s workup depends on (1) the clinical pretest probability of malignancy, (2) nodule morphology, (3) the size and position of the nodule, and (4) the scanning facility available.
Because malignant nodules have increased glucose metabolism compared with benign lesions and healthy lungs, enhancement of the lesion makes it likely to be malignant. Injection of analogue 18-F-2 fluorodeoxyglucose (FDG) is used to assess the metabolic activity. FDG-PET scans may be analyzed semiquantitatively using standardized uptake values (SUVs) to normalize measurements for the patient's weight and the injected dose of radioisotope.
Although visual analysis findings (depending on the experience and judgment of the nuclear medicine physician) may match SUV calculations, an SUV of less than 2.5 is considered indicative of a benign lesion.
FDG-PET scans are quite helpful in detecting mediastinal metastases, thus improving staging of noninvasive lung cancer.
Several studies have reported the sensitivity, specificity, and accuracy of FDG-PET scanning to be greater than 90%, 75%, and 90%, respectively,including a meta-analysis of 40 studies evaluating 1474 focal pulmonary lesions of any size.FDG-PET scanning is an accurate and noninvasive imaging test for the diagnosis of pulmonary nodules and larger masses.However, not much data are available for nodules smaller than 1 cm in diameter.
FDG-PET scans have several limitations because the false-positive findings occur in other metabolically active pulmonary nodules that are either infectious or inflammatory. Moreover, tumors that have lower metabolic rates, such as carcinoid and bronchoalveolar carcinoma, may be difficult to distinguish from background activity. Finally, the FDG-PET scan has lower sensitivity for nodules smaller than 20 mm in diameter and may miss lesions smaller than 10 mm.
One study found that integrated PET-CT scanning is more sensitive and accurate than helical dynamic CT (HDCT) scanning for malignant nodule diagnosis, making it the first-line evaluation tool for solitary pulmonary nodules. The sensitivity, specificity, and accuracy for malignancy with HDCT scanning were 81% (64 of 79 nodules), 93% (37 of 40 nodules), and 85% (101 of 119 nodules), respectively, whereas the values for integrated PET/CT scanning were 96% (76 of 79 nodules), 88% (35 of 40 nodules), and 93% (111 of 119 nodules), respectively.
PET scanning has low sensitivity in small and slow-growing lesions, such as bronchoalveolar carcinoma and carcinoid tumors.One study showed very high false-negative rates (up to 100%) for bronchoalveolar carcinoma.
Because of the high specificity and acceptable sensitivity and accuracy of HDCT scanning, it may be a reasonable alternative if PET-CT scanning is unavailable.
Single-photon emission CT (SPECT) scanning is less expensive than PET scanning, but both modalities have comparable sensitivity and specificity. SPECT imaging is performed using a radiolabeled somatostatin-type receptor binder, technetium-99m ( Tc) P829 (depreotide). SPECT imaging has not been evaluated in a large series of patients; in a smaller series, the sensitivity fell significantly for nodules less than 20 mm in diameter.
In a prospective, multicenter trial, Naalsund et al evaluated the efficacy of Tc depreotide in differentiating benign solitary pulmonary nodules from malignant solitary pulmonary nodules and found that SPECT scanning with Tc depreotide revealed a sensitivity, specificity, and diagnostic accuracy of 89%, 67%, and 81%, respectively. Furthermore, in patients who underwent both Tc depreotide SPECT imaging and FDG-PET imaging, the sensitivity, specificity, and diagnostic accuracy were identical for both modalities.
Rigid and fiberoptic bronchoscopy are each useful for diagnosing endobronchial benign lung tumors. Biopsy or bronchial brushing can be performed with this procedure, as well as excision of a pedunculated endobronchial lesion.
Sensitivity for detection of malignancy is 10-30% when the nodules are peripheral and small (< 2 cm). However, advances in bronchoscopy, such as the development of electromagnetic navigation and endobronchial ultrasonographically guided transbronchial needle biopsy, may offer improved results in the evaluation of pulmonary nodules and mediastinal adenopathy.
Bronchoscopic resection also offers an alternative to surgical resection of benign endobronchial tumors. In a study by Luckraz et al, 100% and 94% of completely resected carcinoids were free of disease at 1 and 10 years, respectively.
A biopsy of a lung nodule should be performed to determine whether it is malignant. Contraindications to a biopsy procedure are bleeding diathesis and cardiopulmonary conditions, which may place the patient's life at risk as a result of the procedure.
Biopsy of a solitary pulmonary nodule can be performed bronchoscopically or via CT-guided transthoracic needle aspiration (TTNA).
Because the yield from bronchoscopy is only 10-20% when the nodule is less than 2 cm in diameter, bronchoscopy and transbronchial needle aspiration (TBNA) may be helpful when the lesion is either endobronchial in location or near a large airway.
Prospective data from the NELSON lung cancer screening trial indicated that the sensitivity of bronchoscopy for suspicious nodules seen on CT scan is only 8.3%. However, if an endobronchial lesion is visualized, the sensitivity increases to 81.8%.TBNA may also be helpful in sampling the mediastinal nodes. Fluoroscopy or endobronchial ultrasonography can be used to localize the lesions during TBNA to increase the diagnostic yield to 70% or more.
TTNA reportedly has an accuracy of 90-95% when the lesion is 2 cm or larger in diameter, although the diagnosis is less accurate (60-80%) in lesions smaller than 2 cm. Confirming a specific benign diagnosis is more difficult (approximately 70% accuracy). Therefore, most benign lesions are characterized as nondiagnostic following TTNA. The rate of pneumothorax following TTNA is approximately 25%, with approximately 7% of patients requiring chest intubation.
A biopsy can be obtained from a superficial, pleurally based lesion, or the lesion can be resected using this approach.
This procedure may occasionally be required when the etiology of a pulmonary nodule is questioned after a thorough workup. Thoracotomy is planned after the appropriate workup and plan of management is decided. The extent of resection is typically decided upon intraoperatively.
In a study of CT scan ̶ guided, transthoracic fine-needle aspiration of pulmonary nodules, Gelbman et al determined that various factors, including nodule size and the occurrence of pneumothorax, influence the rate of false-negative results. The study looked at 170 patients with negative results following fine-needle aspiration, including 18 patients with false-negative findings.
Among the differences found between the two groups of patients, it was determined that those with false negatives had larger nodules (mean, 27 mm) than did those with true-negative results (mean, 17 mm). The false-negative patients also had fewer imaging adjustments per needle pass (4.5) than did the true-negative patients (6.4), as well as a greater pneumothorax rate during the procedure (50% vs 22%).
Because invasive procedures such as TTNA, TBNA, and video-assisted thoracoscopic surgery may be associated with risks and complications, informed consent must be obtained before these procedures are conducted.
A solitary nodule in a young, nonsmoking patient can be monitored with serial radiographs as long as the solitary nodule does not double in size in less than a year and it does not significantly increase in the pattern of calcification and shape consistent with a malignancy.
Lesions that have typical benign features, such as lack of change over two years or a benign pattern of calcification, especially in low-risk patients, do not require further workup. On the other hand, lesions that are strongly suggestive of malignancy (eg, > 3 cm diameter) or those with documented growth should be referred for surgical resection.
Management decisions for lesions with intermediate probability (which is the case for most lesions) are more complex. Although management varies amongst individual institutions and practitioners, several guidelines have been published.
In 2005, the Fleischner Society published guidelinesfor follow-up imaging of solitary pulmonary nodules (SPNs). They specified different strategies based on patient risk factors and the size of the nodule.
According to the guidelines, follow-up imaging for low-risk patients should occur as follows:
The guidelines state that follow-up imaging for high-risk patients should be administered as follows:
The American College of Chest Physicians (ACCP) proposed new guidelines in 2007for the management of solitary pulmonary nodules; these can be summarized as follows:
Management of indeterminate lesions greater than 8-10 mm depends on clinical probability of malignancy, as follows:
Management of pure ground glass lesions or lesions with mixed ground glass and solid components is more controversial and no formal guidelines have been made. Thus careful consideration of available data and clinical judgement should be utilized on a case-by-case basis to manage these lesions.
When a lesion is likely to be malignant, surgical resection, not transthoracic needle aspiration (TTNA) or observation, is often employed. Nonsurgical therapy is limited only to the initial management of complications and associated comorbidity.
Electrocardiographic assessment is required before surgery as part of the preoperative cardiac risk factor assessment. Address the presence of major arrhythmia and ischemia before performing the planned procedure.
The purpose of surgical intervention for benign lung tumors is to avoid missing potentially malignant lesions. Otherwise, benign lung tumors should be removed when they are symptomatic. The existence of symptoms indicates that complications such as pneumonia, atelectasis, and/or hemoptysis are present.
The extent of surgery may be simple endoscopic resection, thoracotomy with bronchotomy/local excision, segmental resection, lobectomy, sleeve resection, or pneumonectomy. The extent of the procedure is usually determined at surgery and is as conservative as possible.
Advances in minimally invasive techniques have made it less important to avoid removing lesions that may be benign. No longer must a patient be subjected to a large incision (posterolateral thoracotomy) for the purpose of diagnosing a solitary pulmonary nodule or treating a benign lung tumor. Moreover, localized resection (wedge resection) performed with a minimally invasive technique has decreased the length of hospital stay and morbidity for patients with benign lung tumors.
Commonly, surgical resection is recommended for bronchial adenomas because of the potential for malignancy. The surgical approach should include complete resection, sparing of as much lung as possible, and lymph node dissection. Endoscopic resection using a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser can be employed for adenoma in high-risk or elderly patients.
Anesthetic preparation is similar to that for any standard thoracotomy and involves the use of an epidural, a double-lumen endotracheal tube, and invasive lines (including a radial artery catheter and a central line). Prior to double-lumen placement, bronchoscopy via a standard endotracheal tube should identify any endobronchial component and plan for the surgical resection.
The 2007 ACCP guidelines recommend that patients who have indeterminate lung nodules with a high probability of malignancy undergo thoracoscopic wedge resections if the lesion is in the peripheral third of the lung. This is because of the relatively low morbidity and mortality associated with the procedure, compared with thoracotomy.If frozen sections show evidence of malignancy, anatomic resection with mediastinal lymph node sampling or dissection may be performed.
Localization using methylene blue injection or wire placement has facilitated successful resection of smaller nodules with video-assisted thoracoscopic surgery. Intraoperative ultrasonography is also suggested as a means of nodule localization during this type of operation.
At the time of open thoracotomy, perform a complete tumor resection and conserve as much lung as possible. In the setting of a lung adenoma, a complete lymph node dissection should also be performed.
For a proven malignant solitary pulmonary nodule, lobectomy is preferred over wedge resection or segmentectomy because of the lower rate of recurrence and a trend toward increased 5-year survival with lobectomy.
Avoiding certain occupational, recreational, and environmental respiratory exposures may help to prevent solitary pulmonary nodule formation. This includes avoidance of risk factors for malignancy, which include smoking and occupational exposures (eg, asbestos, radon, nickel, chromium, vinyl chloride, polycyclic hydrocarbons).
Avoidance of travel to areas endemic for mycosis (eg, histoplasmosis, coccidioidomycosis, blastomycosis) or to areas with a high prevalence of TB can also help to prevent the development of these nodules.
Asif Alavi, MD Resident Physician, Department of Internal Medicine, University of California, Los Angeles, David Geffen School of Medicine, Olive View Medical Center
Asif Alavi, MD is a member of the following medical societies: American College of Physicians
Coauthor(s)
Nader Kamangar, MD, FACP, FCCP, FCCM Professor of Clinical Medicine, University of California, Los Angeles, David Geffen School of Medicine; Chief, Division of Pulmonary and Critical Care Medicine, Vice-Chair, Department of Medicine, Olive View-UCLA Medical Center
Nader Kamangar, MD, FACP, FCCP, FCCM is a member of the following medical societies: American College of Chest Physicians, American College of Physicians, American Lung Association, American Medical Association, American Thoracic Society, Society of Critical Care Medicine, American Academy of Sleep Medicine, American Association for Bronchology and Interventional Pulmonology, World Association for Bronchology and Interventional Pulmonology, Association of Specialty Professors, Clerkship Directors in Internal Medicine, Association of Pulmonary and Critical Care Medicine Program Directors
Chief Editor
Ryland P Byrd, Jr, MD Professor of Medicine, Division of Pulmonary Disease and Critical Care Medicine, James H Quillen College of Medicine, East Tennessee State University
Ryland P Byrd, Jr, MD is a member of the following medical societies: American College of Chest Physicians, American Thoracic Society
Acknowledgements
Oluyinka S Adediji, MD Consulting Staff, Department of Adult and General Medicine, Health Services Incorporated, Montgomery, Alabama
Oluyinka S Adediji, MD is a member of the following medical societies: American College of Physicians and American Medical Association
Disclosure: Nothing to disclose.
John Geibel, MD, DSc, MA Vice Chair and Professor, Department of Surgery, Section of Gastrointestinal Medicine, and Department of Cellular and Molecular Physiology, Yale University School of Medicine; Director, Surgical Research, Department of Surgery, Yale-New Haven Hospital
John Geibel, MD, DSc, MA is a member of the following medical societies: American Gastroenterological Association, American Physiological Society, American Society of Nephrology, Association for Academic Surgery, International Society of Nephrology, New York Academy of Sciences, and Society for Surgery of the Alimentary Tract
Disclosure: AMGEN Royalty Consulting; ARdelyx Ownership interest Board membership
Dale K Mueller, MD Clinical Associate Professor of Surgery, Section Chief, Department of Surgery, University of Illinois College of Medicine; Co-Medical Director, Thoracic Center of Excellence, Vice-Chair, Department of Cardiovascular Medicine and Surgery, OSF St Francis Medical Center; Director, Adult ECMO, Cardiovascular and Thoracic Surgeon, HeartCare Midwest, SC
Dale K Mueller, MD is a member of the following medical societies: American College of Chest Physicians, American College of Surgeons, American Medical Association, American Medical Writers Association, Chicago Medical Society, Illinois State Medical Society, and Society of Thoracic Surgeons
Disclosure: Nothing to disclose.
Sri R Navaratnam, MBBS, PhD, FRCPC Assistant Professor, Department of Internal Medicine, Section of Hematology/Oncology, University of Manitoba; Consulting Medical Oncologist, Department of Hematology/Oncology, Cancer Care Manitoba
Norvin Perez, MD Medical Director, Juneau Urgent and Family Care
Norvin Perez, MD is a member of the following medical societies: American College of Emergency Physicians and American Medical Association
Disclosure: Nothing to disclose.
Stephen P Peters, MD, PhD, FACP, FAAAAI, FCCP, FCPP Professor of Genomics and Personalized Medicine Research, Internal Medicine, and Pediatrics, Associate Director, Center for Genomics and Personalized Medicine Research, Director of Research, Section on Pulmonary, Critical Care, Allergy and Immunologic Diseases, Wake Forest University School of Medicine
Stephen P Peters, MD, PhD, FACP, FAAAAI, FCCP, FCPP is a member of the following medical societies: American Academy of Allergy Asthma and Immunology, American Association of Immunologists, American College of Chest Physicians, American College of Physicians, American Federation for Medical Research, American Thoracic Society, and Sigma Xi
Disclosure: See below for list of all activities None None
Daniel S Schwartz, MD, FACS Assistant Clinical Professor of Cardiothoracic Surgery, Mount Sinai School of Medicine; Chief of Thoracic Surgery, Huntington Hospital
Daniel S Schwartz, MD, FACS is a member of the following medical societies: American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, and Western Thoracic Surgical Association
Disclosure: Nothing to disclose.
Sat Sharma, MD, FRCPC Professor and Head, Division of Pulmonary Medicine, Department of Internal Medicine, University of Manitoba; Site Director, Respiratory Medicine, St Boniface General Hospital
Sat Sharma, MD, FRCPC is a member of the following medical societies: American Academy of Sleep Medicine, American College of Chest Physicians, American College of Physicians-American Society of Internal Medicine, American Thoracic Society, Canadian Medical Association, Royal College of Physicians and Surgeons of Canada, Royal Society of Medicine, Society of Critical Care Medicine, and World Medical Association
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Richard Thurer, MD B and Donald Carlin Professor of Thoracic Surgical Oncology, University of Miami, Leonard M Miller School of Medicine
Richard Thurer, MD is a member of the following medical societies: American Association for Thoracic Surgery, American College of Chest Physicians, American College of Surgeons, American Medical Association, American Thoracic Society, Florida Medical Association, Society of Surgical Oncology, and Society of Thoracic Surgeons
Disclosure: Nothing to disclose.
References
Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.
A 1.5-cm coin lesion in the left upper lobe in a patient with prior colonic carcinoma. Transthoracic needle biopsy findings confirmed this to be a metastatic deposit.
Mediastinal windows of the patient in the previous image
Right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
Close-up view of a right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
Left upper lobe cavitating solitary nodule eventually identified as active pulmonary tuberculosis from percutaneous needle biopsy findings.
A left upper lobe nodule with central lucency and poorly circumscribed margins was diagnosed as actinomycosis based on needle biopsy findings.
Computed tomography (CT) scan of the patient in the previous image. After needle biopsy, the presence of classic sulfur granules confirmed a diagnosis of actinomycosis.
A right lower lobe solitary pulmonary nodule that was later identified as a hamartoma.
Wedge-shaped peripheral (pleural based) density observed secondary to pulmonary infarction (pulmonary embolism). This is termed the Hampton hump.
Left upper lobe 1.5-cm nodule shows negative computed tomography (CT) scan numbers, suggesting fat in the lesion consistent with hamartoma.
A left upper lobe solitary pulmonary nodule. The differential diagnosis in such cases is large, but computed tomography (CT) scan findings help to narrow the differentials and establish the diagnosis.
Cavitating right lower lobe nodule later confirmed to be primary pulmonary lymphoma. Calcium deposits may also be present in the lesion.
This left lower lobe carcinoid tumor was quite bloody after a percutaneous needle biopsy was performed.
Lateral radiograph of the patient in the previous image.
Computed tomography (CT) scan of a patient with a left lower lobe carcinoid tumor shows a well-circumscribed lesion.
A popcorn calcification in the left lung nodule indicates a benign lesion or hamartoma. No further tests or observations were needed for this patient.
A 1.5-cm right upper lobe nodule on a computed tomography (CT) scan was determined to be a benign, fibrous lesion on needle biopsy. A follow-up at 2 years showed no change in the size of this lesion.
The parenchymal lesion in this computed tomography (CT) scan demonstrates low attenuation within the lesion, indicating the presence of fat. Fat density is observed only in hamartoma and lipoid pneumonia. The likely diagnosis is hamartoma
This patient has a low risk for malignancy of the right upper lobe nodule. Therefore, continued observation with repeat chest radiographs to establish a growth pattern is the best treatment option.
Overview
A solitary pulmonary nodule is defined as a discrete, well-marginated, rounded opacity less than or equal to 3 cm in diameter that is completely surrounded by lung parenchyma, does not touch the hilum or mediastinum, and is not associated with adenopathy, atelectasis, or pleural effusion. Lesions larger than 3 cm are considered masses and are treated as malignancies until proven otherwise. (See the images below.)
 Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.
Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.  A 1.5-cm coin lesion in the left upper lobe in a patient with prior colonic carcinoma. Transthoracic needle biopsy findings confirmed this to be a metastatic deposit.
A 1.5-cm coin lesion in the left upper lobe in a patient with prior colonic carcinoma. Transthoracic needle biopsy findings confirmed this to be a metastatic deposit.  Mediastinal windows of the patient in the previous image
Mediastinal windows of the patient in the previous image See The Solitary Pulmonary Nodule: Is It Lung Cancer?, a Critical Images slideshow, for more information on benign and malignant etiologies of solitary pulmonary nodules.
Patients with solitary pulmonary nodules are usually asymptomatic. However, solitary pulmonary nodules can pose a challenge to clinicians and patients. Whether detected serendipitously or during a routine investigation, a nodule on a chest radiograph raises the following questions:
- Is the nodule benign or malignant
- Should it be investigated or observed
- Should it be surgically resected
Most solitary pulmonary nodules are benign. However, they may represent an early stage of lung cancer. Lung cancer is the leading cause of cancer death in the United States, accounting for more deaths annually than breast, colon, and prostate cancers combined. Lung cancer survival rates remain dismally low at 14% at 5 years.
Patients with early lung cancer, when the primary tumor is less than 3 cm in diameter without evidence of lymph node involvement or distant metastasis (stage 1A), have a 5-year survival rate of 70-80%. Therefore, prompt diagnosis and management of early lung cancer manifesting as a solitary pulmonary nodule is the the best chance for cure.
Benign lung tumors
Benign lung tumors are a heterogenous group of neoplastic lesions originating from pulmonary structures. These tumors include bronchial adenomas, hamartomas, and a group of uncommon neoplasms (eg, chondromas, fibromas, lipomas, leiomyomas, hemangiomas, teratomas, pseudolymphomas, endometrioma, and bronchial glomus tumors).
Although benign lung tumors do not pose a significant health problem, complications can result if an obstructive lesion predisposes the patient to pneumonia, atelectasis, and hemoptysis.
Determination of whether a lung nodule is benign or malignant based solely on its anatomical location is an incorrect practice. Anatomical location has no predictability on the malignant potential of a tumor. Benign lung tumors can occur in the periphery of the lung, but they can also occur as endobronchial lesions within the tracheobronchial tree.
Characteristics
Neoplastic lesions are characterized by the autonomous proliferation of cells without a response to the normal control mechanisms governing cell growth. An additional characteristic of benign tumors is extension without local tissue invasion or spread to other sites.
Hamartomas consist of haphazardly organized mature cells and tissues. Hamartomas are composed mostly of masses of hyaline cartilage with a myxoid connective tissue, adipose cells, smooth muscle cells, and clefts lined with respiratory epithelium. (See the image below.)
 A right lower lobe solitary pulmonary nodule that was later identified as a hamartoma.
A right lower lobe solitary pulmonary nodule that was later identified as a hamartoma. Classification
Benign lung tumors can be classified pathologically, but a clinically useful classification would combine location (ie, endobronchial or parenchymal) and information about whether the lesions are single or multiple. Benign lung tumors can also be classified by their presumed origin. Those classifications include the following:
- Unknown - Hamartoma, clear cell, and teratoma
- Epithelial – Papilloma and polyps
- Mesodermal - Fibroma, lipoma, leiomyoma, chondroma, granular cell tumor, and sclerosing hemangioma
- Other - Myofibroblastic tumor, xanthoma, amyloid, and mucosa-associated lymphoid tumor
Adenomas and hamartomas constitute the largest group of benign lung tumors and, thus, deserve detailed descriptions.
Nodule growth
Generally, a pulmonary nodule must reach 1 cm in diameter before it can be identified on a chest radiograph. For a malignant nodule to reach this size, approximately 30 doublings would have occurred. The average doubling time for a malignant tumor is 120 days (range 7-590 d). A lesion at this growth rate may be present for 10 years before discovery.
Patient education
For patient education information, see the Cancer Center, as well as Bronchoscopy and Bronchial Adenoma.
Types of Benign Pulmonary Tumors
A solitary pulmonary nodule may be secondary to a wide differential of causes. However, more than 95% are malignancies (most likely primary), granulomas (most likely infectious), or benign tumors (most likely hamartomas).
The cause and pathogenesis of benign lung tumors are poorly understood. The nomenclature of benign lung tumors is based on histologic findings. Some of these tumors have benign features, while others are hamartomas.
Hamartomas
Hamartomas (chondroadenomas) are the most common type of benign lung tumor. They occur primarily in adults, although they do occasionally arise in children. Hamartomas are peripherally located. Grossly, they have a firm, marblelike consistency. Histologically, hamartomas generally consist of epithelial tissue and other tissues, such as fat and cartilage. Hamartomas can be easily enucleated, but wedge resection is also appropriate.
Bronchial adenomas
Bronchial adenomas make up 50% of all benign pulmonary tumors. The term bronchial adenoma should be discouraged because, when used loosely, it includes carcinoid tumors, adenocystic carcinomas, and mucoepidermoid carcinomas, which, in fact, are low-grade malignant tumors.
Mucous gland adenomas
Mucous gland adenomas are true benign bronchial adenomas. Mucous gland adenomas, which are also called bronchial cystadenomas, arise in the main or local bronchi. Histologically, they consist of columnar cell–lined cystic spaces with a papillary appearance.
Tracheobronchial tumors
Multiple laryngeal papillomatosis is a viral disease of the upper airway that primarily affects children. This disorder has malignant potential and may later spread to the tracheobronchial tree.
Solitary papillomas usually are less than 1.5 cm in diameter. They usually are lobar or segmental in location and are histologically similar to viral papillomatosis.
Inflammatory papilloma is a solitary polypoid mass of granulation tissue that is associated with an underlying pulmonary inflammatory condition.
Granular cell myoblastomas are of neural cell origin. A granular cell myoblastoma contains polygonal or spindle cells with granular cytoplasm. Granular cell myoblastomas tend to be multiple in 10% of cases and are more common in men aged 30-50 years.
Other parenchymal tumors occasionally occurring in the endobronchial tree (eg, leiomyoma, lipoma) almost exclusively are found at an endobronchial location.
Sclerosing hemangiomas
Sclerosing hemangioma is an uncommon tumor derived from the epithelial cells of pneumocytes (terminal bronchiolar cells). This tumor consists of several elements, including solid cellular areas, papillary structures, sclerotic regions, and blood-filled spaces. Sclerosing hemangiomas are most commonly found in middle-aged women. Chest radiography demonstrates a well-defined nodule that is less than 3 cm.
Other mesenchymal tumors include lipoma, leiomyoma, neural tumors, fibroma, benign clear-cell tumor, teratoma, plasma cell granuloma, fibrous histiocytoma, xanthoma, pulmonary hyalinizing granuloma, pulmonary endometrioma, and pseudolymphoma.
Multiple tumors
Many benign lung tumors occasionally have multiple origins. Among these are hamartomas, hyalinizing granulomas, leiomyomas, and sclerosing hemangiomas.
The Carney triad is a syndrome of gastric epithelioid leiomyosarcoma, pulmonary chondromas, and extra-adrenal paragangliomas. The Carney triad mainly affects women.
Pulmonary tumorlets are minute collections of neuroendocrine cells scattered throughout the lung. Pulmonary tumorlets predominantly affect older women.
Clinically significant intrapulmonary chemodectomas apparently are paragangliomas. They behave in a benign fashion.
Etiology
Bearing in mind that the major distinction that must be made is between neoplastic and inflammatory lesions, solitary pulmonary nodules have several causes:
Neoplastic (malignant or benign) tumors can be caused by the following:
- Bronchogenic carcinoma
- Adenocarcinoma (including bronchoalveolar carcinoma)
- Squamous cell carcinoma
- Large cell lung carcinoma
- Small cell lung cancer
- Metastasis
- Lymphoma
- Carcinoid
- Hamartoma
- Connective-tissue and neural tumors - Fibroma, neurofibroma, blastoma, and sarcoma
Inflammatory (infectious) nodules can result from the following:
- Granuloma - Tuberculosis (TB), histoplasmosis, coccidioidomycosis, blastomycosis, cryptococcosis, and nocardiosis
- Lung abscess
- Round pneumonia
- Hydatid cyst
Inflammatory (noninfectious) nodules can be caused by the following:
- Rheumatoid arthritis
- Wegener granulomatosis
- Sarcoidosis
- Lipoid pneumonia
Congenital nodules can be produced by the following:
- Arteriovenous malformation
- Pulmonary sequestration
- Bronchogenic cyst
Other causes of pulmonary nodules include the following:
- Pulmonary infarct
- Rounded atelectasis
- Mucoid impaction
- Progressive massive fibrosis
Epidemiology
Occurrence in the United States
Solitary pulmonary nodules are one of the most common thoracic radiographic abnormalities. Approximately 150,000 cases are detected each year as an incidental finding, either on chest radiographs or on thoracic computed tomography (CT) scans.In lung cancer screening studies that enrolled people at high risk for lung cancer, the prevalence of solitary pulmonary nodules ranged from 8-51%.
Approximately 40-50% of solitary pulmonary nodules are malignant. Gould et al reported after a review of the literature that most of these are adenocarcinoma (47%), followed by squamous cell carcinoma (22%). Small cell lung cancer makes up only 4% of malignant solitary pulmonary nodules.
Sex- and age-related demographics
Reported series suggest that benign lung tumors affect men more frequently than women (adenoma and hamartoma).
Risk of malignancy increases with age. For individuals younger than 39 years, the risk is 3%. The risk increases to 15% for individuals aged 40-49 years, to 43% for persons aged 50-59 years, and to more than 50% for persons older than 60 years.
Prognosis
Surgical resection is curative for most benign lung tumors. The 5- and 10-year survival rates following surgical resection of typical carcinoid tumors of the lung are 95% and 90%, respectively. The 5- and 10-year survival rates for patients with atypical carcinoids are 40-70% and 18-50%, respectively.
In one study, complete bronchoscopic resection for endobronchial carcinoid tumors at 1 and 10 years provided disease-free states at rates of 100% and 94%, respectively. (See the images below.)
 This left lower lobe carcinoid tumor was quite bloody after a percutaneous needle biopsy was performed.
This left lower lobe carcinoid tumor was quite bloody after a percutaneous needle biopsy was performed.  Lateral radiograph of the patient in the previous image.
Lateral radiograph of the patient in the previous image.  Computed tomography (CT) scan of a patient with a left lower lobe carcinoid tumor shows a well-circumscribed lesion.
Computed tomography (CT) scan of a patient with a left lower lobe carcinoid tumor shows a well-circumscribed lesion. Although most solitary pulmonary nodules are benign, they may represent an early stage of lung cancer. While lung cancer survival rates remain dismally low at 14% at 5 years, diagnosis of early lung cancer (ie, when the primary tumor has a diameter smaller than 3 cm with no lymph node involvement and no distant metastasis [stage 1A]) can be associated with a 5-year survival rate of 70-80%. Accordingly, the best chance for cure of early lung cancer manifesting as a solitary pulmonary nodule is prompt diagnosis and management.
Possible complications due to benign lung tumors include pneumonia, atelectasis, hemoptysis, hyperinflation, and malignancy.
Patient History
Most patients with solitary pulmonary nodules are asymptomatic; the nodules are typically detected as an incidental finding. Approximately 20-30% of all bronchogenic carcinomas appear as solitary pulmonary nodules on initial radiographs. The following features are important when assessing whether the nodule is benign or malignant:
- History of malignancy
- History of smoking
- Occupational risk factors for lung cancer - Exposure to asbestos, radon, nickel, chromium, vinyl chloride, and polycyclic hydrocarbons can lead to the development of a solitary pulmonary nodule
- Travel to areas with endemic mycosis (eg, histoplasmosis, coccidioidomycosis, blastomycosis) or with a high prevalence of TB can lead to the development of a benign solitary pulmonary nodule
- History of TB or pulmonary mycosis
Physical Examination
The diagnostic approach is basically the same for these tumors, with clinical presentations depending on the location of the tumor (eg, trachea, other airways, parenchyma). Hemoptysis, lung mass on chest radiograph, and unresolved infiltrates may be present. Symptoms may include the following:
- Pseudoasthmatic (localized) wheezing
- Persistent coughing
- Shortness of breath
- Hemoptysis
- Fever - Especially when associated pneumonia is present
- Diminished breath sounds
- Dullness to percussion
- Rales
Most patients are asymptomatic from their solitary pulmonary nodules. If a patient is asymptomatic, the tumor is typically identified serendipitously.
Differential Diagnosis
The following conditions should be considered in the differential diagnosis of a solitary pulmonary nodule:
- Arteriovenous malformation
- Aspergillosis
- Atelectasis
- Blastomycosis
- Carcinoid lung tumors
- Coccidioidomycosis
- Histoplasmosis
- Hydatid cysts
- Lung abscess
- Non-small cell lung cancer
- Oat cell (small cell) lung cancer
- Nocardiosis
- Pancoast tumor
- Rheumatoid arthritis
- Sarcoidosis
- Tuberculosis
- Wegener granulomatosis
Assessing the Probability of Malignancy
Because a malignancy may be curable when present as a solitary pulmonary nodule, great care should be taken in evaluating such lesions. A comprehensive assessment generally includes history, physical examination, evaluation of previous chest radiographs, incorporation of other imaging studies (eg, CT scanning, positron-emission tomography [PET] scanning, single-photon emission CT [SPECT] scanning), and invasive diagnostic procedures.
Because determining the pretest probability of malignancy is essential in guiding the management of solitary pulmonary nodules, estimating the probability of benignity using a validated quantitative model might be an effective strategy. Bayesian analysis combines the radiologic features of a nodule and the clinical findings of an individual patient to estimate the probability of malignancy.
The radiographic features of solitary pulmonary nodules included in the Bayes analysis are size, edge, contour, cavity-wall thickness, and growth rate; the clinical parameters are smoking, age, previous malignancy, and hemoptysis. (These features and parameters have been derived from previously published series.)
The calculation of likelihood ratios and their use may result in fewer false-negative and false-positive results.
Because the evidence is not definitive for many of the management guidelines, clinicians should discuss with patients the risks and benefits of alternative management options and should elicit patient preferences. The probability of malignancy only provides an estimate based on previously published studies and may not be generalized to an individual patient. Therefore, patient preferences and clinician experience are important in planning further management strategies.
Laboratory Studies
Laboratory studies have a limited role in the workup of solitary pulmonary nodules. Anemia or an elevated sedimentation rate may indicate an underlying neoplastic or infectious process, while elevated levels of liver enzymes, alkaline phosphatase, or serum calcium may indicate metastases from a solitary bronchogenic carcinoma or from a nonpulmonary malignancy.
Patients who have histoplasmosis or coccidioidomycosis may have high levels of immunoglobulin G and immunoglobulin M antibodies specific to these fungi.
Preoperative tests
A preoperative complete blood cell count should be performed on all patients who undergo tissue sampling. The cell count also helps to determine the general health status of the patient and in the diagnosis of complications such as pneumonia and anemia.
Coagulation (PT and INR, PTT) studies are recommended before the patient undergoes any invasive procedures. The adequacy of the platelet function should be determined only in selected patients.
Electrolytes and renal and liver function tests help to evaluate the presence of an abnormality that may indicate the need for either intervention or further workup before an invasive procedure is performed.
A tuberculin skin test and sputum cytologic and microbiological studies should also be performed in selected patients.
A patient with a carcinoid tumor, with or without carcinoid syndrome, may exhibit a high level of serotonin and 5-hydroxyindoleacetic acid (5-HIAA).
Arterial blood gas and pulmonary function tests (PFTs) are indicated in patients presenting with shortness of breath and are indicated before invasive procedures or thoracotomy. The presence of hypoxia and hypercarbia generally suggests poor tolerability for resective surgery. PFTs are useful tests when determining patients' suitability for lung resection. Patients must have satisfactory parameters as measured by forced expiratory volume in 1 second (FEV1) and diffusion capacity of lung for carbon monoxide (DLCO).
Chest Radiography and CT Scanning
Because solitary pulmonary nodules are typically first detected on chest radiographs, the initial distinction made is whether the nodule is pulmonary or extrapulmonary in nature. Findings from lateral chest radiography, fluoroscopy, or CT scanning may help to confirm the location of the nodule. Although nodules of 5 mm in diameter are occasionally visualized on chest radiographs, solitary pulmonary nodules are quite often 8-10 mm in diameter.
Chest radiographs can provide information regarding size, shape, cavitation, growth rate, and calcification pattern. All of these radiologic features can help in determining whether the lesion is benign or malignant. However, none of these features is entirely specific for lung carcinoma.
CT scanning of the chest has many advantages over plain chest radiography.The advantages include better resolution of nodules and detection of nodules as small as 3-4 mm. CT scan images also help to better characterize the morphologic features of various lesions. Multiple nodules and regions that are difficult to assess on chest radiographs are better visualized on CT scan images. (See the images below.)
 A left upper lobe nodule with central lucency and poorly circumscribed margins was diagnosed as actinomycosis based on needle biopsy findings.
A left upper lobe nodule with central lucency and poorly circumscribed margins was diagnosed as actinomycosis based on needle biopsy findings.  Computed tomography (CT) scan of the patient in the previous image. After needle biopsy, the presence of classic sulfur granules confirmed a diagnosis of actinomycosis.
Computed tomography (CT) scan of the patient in the previous image. After needle biopsy, the presence of classic sulfur granules confirmed a diagnosis of actinomycosis.  A left upper lobe solitary pulmonary nodule. The differential diagnosis in such cases is large, but computed tomography (CT) scan findings help to narrow the differentials and establish the diagnosis.
A left upper lobe solitary pulmonary nodule. The differential diagnosis in such cases is large, but computed tomography (CT) scan findings help to narrow the differentials and establish the diagnosis. CT densitometry measures the attenuation coefficients of a lesion and aids detection of occult calcification. Attenuation coefficients are expressed in Hounsfield units (HU); a value of more than 185 HU has been suggested as a cutoff for benign lesions. However, prospective studies have indicated low sensitivity and specificity for CT densitometry measurements. Thus, these measurements are no longer routinely employed.
With regard to dynamic contrast enhancement, a greater degree of contrast enhancement on repeated measurements of attenuation indicates that the nodule is malignant. Enhancement of greater than 20 HU is associated with malignancy, whereas less than 15 HU suggests a benign lesion. A multicenter study using a cutoff value of 15 HU found a sensitivity and specificity of 98% and 58%, respectively.Active granulomas or other infectious lesions may also enhance, limiting the application of this technique. However, a failure to enhance by more than 15-20 HU has a greater than a 95% predictive value for benignity.
Several radiologic characteristics found on CT scanning and radiography (although CT scanning is superior) may help to establish the diagnosis or suggest whether a lesion is benign or malignant. These include the following:
- Size
- Growth rate
- Presence of calcification
- Border characteristics
- Internal characteristics
- Location
Size
Although a well-defined nodule of smaller size that is clearly visible on chest radiographs may be calcified and benign, small lesions may very well be early stage bronchogenic carcinoma. A lesion greater than 4 cm in diameter is very likely a bronchogenic carcinoma, although exceptions include lung abscess, Wegener's granulomatosis, lymphoma, round pneumonia, rounded atelectasis, and hydatid cyst.
Midthun et al reported that the likelihood of malignancy was 50% for nodules greater than 20 mm and 18% for those 8-20 mm in diameter. With lesions smaller than 8 mm, a sharp decline is noted, with nodules of 4-7 mm having a likelihood of malignancy of only 0.9% and those less that 3 mm, only 0.2%.
Rate of growth
Serial chest radiographs facilitate estimation of the growth rate of a nodule. Growth rate refers to the doubling time of a nodule, which is a doubling of the nodule volume. Because a nodule on a chest radiograph is seen as a 2-dimensional (2-D) circle rather than a 3-D sphere, an increase in diameter of 26% corresponds to a doubling of nodule volume.
Bronchogenic carcinoma generally doubles in 1-18 months (average 4-8 mo). Although a doubling time of less than 1 month or longer than 18 months makes bronchogenic carcinoma unlikely, it does not exclude the diagnosis completely. Important exceptions are bronchoalveolar carcinoma, which may require more than 2 years to double in size, and metastases from specific tumors (eg, osteosarcoma) that grow rapidly.
In general, doubling times of less than 1 month suggest infections; doubling times of more than 18 months suggest benign processes such as granuloma, hamartoma, bronchial carcinoid, and rounded atelectasis. If a nodule remains the same size for 2 years, it is very likely benign. However, further follow-up monitoring may be indicated.
In one retrospective series, a volume doubling time of less than 400 days at 3 months and 1 year follow-up was strongly predictive of malignancy.
Calcification
Chest radiographs may demonstrate calcification, which often indicates that the lesion is benign. (CT scanning is the most sensitive technique for detection of calcification.) The 5 patterns of calcification usually observed in benign lesions are diffuse, central, laminar, concentric, and popcorn. A stippled or eccentric pattern is associated with malignancy. (See the images below.)
 Right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
Right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.  Close-up view of a right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
Close-up view of a right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.  A popcorn calcification in the left lung nodule indicates a benign lesion or hamartoma. No further tests or observations were needed for this patient.
A popcorn calcification in the left lung nodule indicates a benign lesion or hamartoma. No further tests or observations were needed for this patient.  Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.
Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule. Border characteristics
A very irregular edge or corona radiata (numerous strands radiating into the surrounding lung) may indicate a bronchogenic carcinoma. Whereas lobulation and notching may indicate bronchogenic carcinoma, a well-defined, smooth, nonlobulated edge may indicate a benign lesion or metastasis.
Cavitation with a thin, smooth wall may indicate lung abscess or a benign lesion, whereas thick-walled cavitations imply an underlying malignant neoplasm. (See the images below.)
 Left upper lobe cavitating solitary nodule eventually identified as active pulmonary tuberculosis from percutaneous needle biopsy findings.
Left upper lobe cavitating solitary nodule eventually identified as active pulmonary tuberculosis from percutaneous needle biopsy findings.  Cavitating right lower lobe nodule later confirmed to be primary pulmonary lymphoma. Calcium deposits may also be present in the lesion.
Cavitating right lower lobe nodule later confirmed to be primary pulmonary lymphoma. Calcium deposits may also be present in the lesion. The CT-scan halo sign (ie, ground-glass attenuation surrounding a nodule on CT scan image) most commonly indicates infection with an invasive Aspergillus species. Other, less common possibilities include TB, cytomegalovirus infection, and herpes simplex infections.
Internal characteristics
Several characteristics within the nodule itself can indicate a specific cause. For example, demonstration of fat within the lesion is specific for a hamartoma, a benign lesion. (See the images below.)
 Left upper lobe 1.5-cm nodule shows negative computed tomography (CT) scan numbers, suggesting fat in the lesion consistent with hamartoma.
Left upper lobe 1.5-cm nodule shows negative computed tomography (CT) scan numbers, suggesting fat in the lesion consistent with hamartoma.  The parenchymal lesion in this computed tomography (CT) scan demonstrates low attenuation within the lesion, indicating the presence of fat. Fat density is observed only in hamartoma and lipoid pneumonia. The likely diagnosis is hamartoma
The parenchymal lesion in this computed tomography (CT) scan demonstrates low attenuation within the lesion, indicating the presence of fat. Fat density is observed only in hamartoma and lipoid pneumonia. The likely diagnosis is hamartoma Ground-glass opacities may represent a benign lesion, such as atypical adenomatous hyperplasia, or malignancy, such as bronchoalveolar carcinoma (adenocarcinoma in situ).Importantly, malignant ground-glass opacities often grow slower and may require longer follow-up.
Subsolid nodules (nodules with both a solid and a ground-glass component) are frequently peripheral adenocarcinomas of the lung. Studies have demonstrated excellent correlation between the Noguchi classification of adenocarcinomas and CT-scan findings. Specifically, atypical alveolar hyperplasia typically manifests as pure ground-glass lesions of less than 5 cm; bronchoalveolar carcinoma is usually greater than 5 cm. Lesions with a mixed solid component and ground glass correlate with adenocarcinoma, mixed subtype.
The presence of air bronchograms within the solitary pulmonary nodule makes bronchogenic carcinoma or metastasis unlikely, although they may be observed with bronchoalveolar carcinoma or lymphoma. Invasion of the adjacent bone by the nodule is pathognomic of bronchogenic carcinoma.
Location
Nodules that are attached to pleura, vessels, or fissures are likely to be benign.
Positron-Emission Tomography
Whether positron-emission tomography (PET) scanning will be useful in a patient’s workup depends on (1) the clinical pretest probability of malignancy, (2) nodule morphology, (3) the size and position of the nodule, and (4) the scanning facility available.
Because malignant nodules have increased glucose metabolism compared with benign lesions and healthy lungs, enhancement of the lesion makes it likely to be malignant. Injection of analogue 18-F-2 fluorodeoxyglucose (FDG) is used to assess the metabolic activity. FDG-PET scans may be analyzed semiquantitatively using standardized uptake values (SUVs) to normalize measurements for the patient's weight and the injected dose of radioisotope.
Although visual analysis findings (depending on the experience and judgment of the nuclear medicine physician) may match SUV calculations, an SUV of less than 2.5 is considered indicative of a benign lesion.
FDG-PET scans are quite helpful in detecting mediastinal metastases, thus improving staging of noninvasive lung cancer.
Sensitivity and specificity
Several studies have reported the sensitivity, specificity, and accuracy of FDG-PET scanning to be greater than 90%, 75%, and 90%, respectively,including a meta-analysis of 40 studies evaluating 1474 focal pulmonary lesions of any size.FDG-PET scanning is an accurate and noninvasive imaging test for the diagnosis of pulmonary nodules and larger masses.However, not much data are available for nodules smaller than 1 cm in diameter.
FDG-PET scans have several limitations because the false-positive findings occur in other metabolically active pulmonary nodules that are either infectious or inflammatory. Moreover, tumors that have lower metabolic rates, such as carcinoid and bronchoalveolar carcinoma, may be difficult to distinguish from background activity. Finally, the FDG-PET scan has lower sensitivity for nodules smaller than 20 mm in diameter and may miss lesions smaller than 10 mm.
One study found that integrated PET-CT scanning is more sensitive and accurate than helical dynamic CT (HDCT) scanning for malignant nodule diagnosis, making it the first-line evaluation tool for solitary pulmonary nodules. The sensitivity, specificity, and accuracy for malignancy with HDCT scanning were 81% (64 of 79 nodules), 93% (37 of 40 nodules), and 85% (101 of 119 nodules), respectively, whereas the values for integrated PET/CT scanning were 96% (76 of 79 nodules), 88% (35 of 40 nodules), and 93% (111 of 119 nodules), respectively.
PET scanning has low sensitivity in small and slow-growing lesions, such as bronchoalveolar carcinoma and carcinoid tumors.One study showed very high false-negative rates (up to 100%) for bronchoalveolar carcinoma.
Because of the high specificity and acceptable sensitivity and accuracy of HDCT scanning, it may be a reasonable alternative if PET-CT scanning is unavailable.
SPECT Scanning
Single-photon emission CT (SPECT) scanning is less expensive than PET scanning, but both modalities have comparable sensitivity and specificity. SPECT imaging is performed using a radiolabeled somatostatin-type receptor binder, technetium-99m ( Tc) P829 (depreotide). SPECT imaging has not been evaluated in a large series of patients; in a smaller series, the sensitivity fell significantly for nodules less than 20 mm in diameter.
In a prospective, multicenter trial, Naalsund et al evaluated the efficacy of Tc depreotide in differentiating benign solitary pulmonary nodules from malignant solitary pulmonary nodules and found that SPECT scanning with Tc depreotide revealed a sensitivity, specificity, and diagnostic accuracy of 89%, 67%, and 81%, respectively. Furthermore, in patients who underwent both Tc depreotide SPECT imaging and FDG-PET imaging, the sensitivity, specificity, and diagnostic accuracy were identical for both modalities.
Fiberoptic Bronchoscopy
Rigid and fiberoptic bronchoscopy are each useful for diagnosing endobronchial benign lung tumors. Biopsy or bronchial brushing can be performed with this procedure, as well as excision of a pedunculated endobronchial lesion.
Sensitivity for detection of malignancy is 10-30% when the nodules are peripheral and small (< 2 cm). However, advances in bronchoscopy, such as the development of electromagnetic navigation and endobronchial ultrasonographically guided transbronchial needle biopsy, may offer improved results in the evaluation of pulmonary nodules and mediastinal adenopathy.
Bronchoscopic resection also offers an alternative to surgical resection of benign endobronchial tumors. In a study by Luckraz et al, 100% and 94% of completely resected carcinoids were free of disease at 1 and 10 years, respectively.
Biopsy
A biopsy of a lung nodule should be performed to determine whether it is malignant. Contraindications to a biopsy procedure are bleeding diathesis and cardiopulmonary conditions, which may place the patient's life at risk as a result of the procedure.
Biopsy of a solitary pulmonary nodule can be performed bronchoscopically or via CT-guided transthoracic needle aspiration (TTNA).
Because the yield from bronchoscopy is only 10-20% when the nodule is less than 2 cm in diameter, bronchoscopy and transbronchial needle aspiration (TBNA) may be helpful when the lesion is either endobronchial in location or near a large airway.
Prospective data from the NELSON lung cancer screening trial indicated that the sensitivity of bronchoscopy for suspicious nodules seen on CT scan is only 8.3%. However, if an endobronchial lesion is visualized, the sensitivity increases to 81.8%.TBNA may also be helpful in sampling the mediastinal nodes. Fluoroscopy or endobronchial ultrasonography can be used to localize the lesions during TBNA to increase the diagnostic yield to 70% or more.
TTNA reportedly has an accuracy of 90-95% when the lesion is 2 cm or larger in diameter, although the diagnosis is less accurate (60-80%) in lesions smaller than 2 cm. Confirming a specific benign diagnosis is more difficult (approximately 70% accuracy). Therefore, most benign lesions are characterized as nondiagnostic following TTNA. The rate of pneumothorax following TTNA is approximately 25%, with approximately 7% of patients requiring chest intubation.
Video-assisted thoracoscopy
A biopsy can be obtained from a superficial, pleurally based lesion, or the lesion can be resected using this approach.
Open biopsy
This procedure may occasionally be required when the etiology of a pulmonary nodule is questioned after a thorough workup. Thoracotomy is planned after the appropriate workup and plan of management is decided. The extent of resection is typically decided upon intraoperatively.
Risk factors for false-negative results
In a study of CT scan ̶ guided, transthoracic fine-needle aspiration of pulmonary nodules, Gelbman et al determined that various factors, including nodule size and the occurrence of pneumothorax, influence the rate of false-negative results. The study looked at 170 patients with negative results following fine-needle aspiration, including 18 patients with false-negative findings.
Among the differences found between the two groups of patients, it was determined that those with false negatives had larger nodules (mean, 27 mm) than did those with true-negative results (mean, 17 mm). The false-negative patients also had fewer imaging adjustments per needle pass (4.5) than did the true-negative patients (6.4), as well as a greater pneumothorax rate during the procedure (50% vs 22%).
Patient consent
Because invasive procedures such as TTNA, TBNA, and video-assisted thoracoscopic surgery may be associated with risks and complications, informed consent must be obtained before these procedures are conducted.
Management Recommendations
A solitary nodule in a young, nonsmoking patient can be monitored with serial radiographs as long as the solitary nodule does not double in size in less than a year and it does not significantly increase in the pattern of calcification and shape consistent with a malignancy.
Lesions that have typical benign features, such as lack of change over two years or a benign pattern of calcification, especially in low-risk patients, do not require further workup. On the other hand, lesions that are strongly suggestive of malignancy (eg, > 3 cm diameter) or those with documented growth should be referred for surgical resection.
Management decisions for lesions with intermediate probability (which is the case for most lesions) are more complex. Although management varies amongst individual institutions and practitioners, several guidelines have been published.
Fleischner Society recommendations
In 2005, the Fleischner Society published guidelinesfor follow-up imaging of solitary pulmonary nodules (SPNs). They specified different strategies based on patient risk factors and the size of the nodule.
According to the guidelines, follow-up imaging for low-risk patients should occur as follows:
- Less than or equal to 4 mm - No further investigation
- 4-6 mm - CT scanning at 12 months
- 6-8 mm - CT scanning at 6-12 months and 18-24 months
- Greater than 8 mm - CT scanning at 3, 9, and 24 months; contrast-enhanced CT scanning; positron-emission tomography (PET) scanning; and/or biopsy
The guidelines state that follow-up imaging for high-risk patients should be administered as follows:
- Less than or equal to 4 mm - CT scanning at 12 months
- 4-6 mm - CT scanning at 6-12 months and 18-24 months
- 6-8 mm - CT scanning at 3-6 months, 9-12 months, and 24 months
- Greater than 8 mm - Same as low-risk patients
ACCP recommendations
The American College of Chest Physicians (ACCP) proposed new guidelines in 2007for the management of solitary pulmonary nodules; these can be summarized as follows:
- Carefully calculate pretest probability for malignancy, either through experienced clinical judgment or through the use of a validated model, such as Bayesian analysis
- Previous chest radiographs should be reviewed to determine if the lesion has been stable over 2 years; if so, no further follow up is necessary, with the exception of pure ground-glass lesions on CT scans, which can be slower growing
- For lesions with a benign pattern of calcification, further testing is not necessary
Management of indeterminate lesions greater than 8-10 mm depends on clinical probability of malignancy, as follows:
- Low probability - Serial CT scanning at 3, 6, 12, and 24 months
- Intermediate probability - FDG-PET scanning, contrast-enhanced CT scanning, transthoracic needle aspiration (TTNA), and/or transbronchial needle aspiration (TBNA) (thoracoscopic diagnosis is recommended for patients who wish to have a surgical diagnosis if the lesion is in the peripheral third of the lung.)
- High probability - Surgical resection
- Subcentimeter lesions - Same recommendations as those by the Fleischner Society, as described above
- Any unequivocal growth noted during follow up - Definitive tissue diagnosis needed
Management of pure ground glass lesions or lesions with mixed ground glass and solid components is more controversial and no formal guidelines have been made. Thus careful consideration of available data and clinical judgement should be utilized on a case-by-case basis to manage these lesions.
Tumor Resection
When a lesion is likely to be malignant, surgical resection, not transthoracic needle aspiration (TTNA) or observation, is often employed. Nonsurgical therapy is limited only to the initial management of complications and associated comorbidity.
Electrocardiographic assessment is required before surgery as part of the preoperative cardiac risk factor assessment. Address the presence of major arrhythmia and ischemia before performing the planned procedure.
The purpose of surgical intervention for benign lung tumors is to avoid missing potentially malignant lesions. Otherwise, benign lung tumors should be removed when they are symptomatic. The existence of symptoms indicates that complications such as pneumonia, atelectasis, and/or hemoptysis are present.
Types of procedures
The extent of surgery may be simple endoscopic resection, thoracotomy with bronchotomy/local excision, segmental resection, lobectomy, sleeve resection, or pneumonectomy. The extent of the procedure is usually determined at surgery and is as conservative as possible.
Advances in minimally invasive techniques have made it less important to avoid removing lesions that may be benign. No longer must a patient be subjected to a large incision (posterolateral thoracotomy) for the purpose of diagnosing a solitary pulmonary nodule or treating a benign lung tumor. Moreover, localized resection (wedge resection) performed with a minimally invasive technique has decreased the length of hospital stay and morbidity for patients with benign lung tumors.
Commonly, surgical resection is recommended for bronchial adenomas because of the potential for malignancy. The surgical approach should include complete resection, sparing of as much lung as possible, and lymph node dissection. Endoscopic resection using a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser can be employed for adenoma in high-risk or elderly patients.
Operative considerations
Anesthetic preparation is similar to that for any standard thoracotomy and involves the use of an epidural, a double-lumen endotracheal tube, and invasive lines (including a radial artery catheter and a central line). Prior to double-lumen placement, bronchoscopy via a standard endotracheal tube should identify any endobronchial component and plan for the surgical resection.
The 2007 ACCP guidelines recommend that patients who have indeterminate lung nodules with a high probability of malignancy undergo thoracoscopic wedge resections if the lesion is in the peripheral third of the lung. This is because of the relatively low morbidity and mortality associated with the procedure, compared with thoracotomy.If frozen sections show evidence of malignancy, anatomic resection with mediastinal lymph node sampling or dissection may be performed.
Localization using methylene blue injection or wire placement has facilitated successful resection of smaller nodules with video-assisted thoracoscopic surgery. Intraoperative ultrasonography is also suggested as a means of nodule localization during this type of operation.
At the time of open thoracotomy, perform a complete tumor resection and conserve as much lung as possible. In the setting of a lung adenoma, a complete lymph node dissection should also be performed.
For a proven malignant solitary pulmonary nodule, lobectomy is preferred over wedge resection or segmentectomy because of the lower rate of recurrence and a trend toward increased 5-year survival with lobectomy.
Prevention
Avoiding certain occupational, recreational, and environmental respiratory exposures may help to prevent solitary pulmonary nodule formation. This includes avoidance of risk factors for malignancy, which include smoking and occupational exposures (eg, asbestos, radon, nickel, chromium, vinyl chloride, polycyclic hydrocarbons).
Avoidance of travel to areas endemic for mycosis (eg, histoplasmosis, coccidioidomycosis, blastomycosis) or to areas with a high prevalence of TB can also help to prevent the development of these nodules.
Asif Alavi, MD Resident Physician, Department of Internal Medicine, University of California, Los Angeles, David Geffen School of Medicine, Olive View Medical Center
Asif Alavi, MD is a member of the following medical societies: American College of Physicians
Coauthor(s)
Nader Kamangar, MD, FACP, FCCP, FCCM Professor of Clinical Medicine, University of California, Los Angeles, David Geffen School of Medicine; Chief, Division of Pulmonary and Critical Care Medicine, Vice-Chair, Department of Medicine, Olive View-UCLA Medical Center
Nader Kamangar, MD, FACP, FCCP, FCCM is a member of the following medical societies: American College of Chest Physicians, American College of Physicians, American Lung Association, American Medical Association, American Thoracic Society, Society of Critical Care Medicine, American Academy of Sleep Medicine, American Association for Bronchology and Interventional Pulmonology, World Association for Bronchology and Interventional Pulmonology, Association of Specialty Professors, Clerkship Directors in Internal Medicine, Association of Pulmonary and Critical Care Medicine Program Directors
Chief Editor
Ryland P Byrd, Jr, MD Professor of Medicine, Division of Pulmonary Disease and Critical Care Medicine, James H Quillen College of Medicine, East Tennessee State University
Ryland P Byrd, Jr, MD is a member of the following medical societies: American College of Chest Physicians, American Thoracic Society
Acknowledgements
Oluyinka S Adediji, MD Consulting Staff, Department of Adult and General Medicine, Health Services Incorporated, Montgomery, Alabama
Oluyinka S Adediji, MD is a member of the following medical societies: American College of Physicians and American Medical Association
Disclosure: Nothing to disclose.
John Geibel, MD, DSc, MA Vice Chair and Professor, Department of Surgery, Section of Gastrointestinal Medicine, and Department of Cellular and Molecular Physiology, Yale University School of Medicine; Director, Surgical Research, Department of Surgery, Yale-New Haven Hospital
John Geibel, MD, DSc, MA is a member of the following medical societies: American Gastroenterological Association, American Physiological Society, American Society of Nephrology, Association for Academic Surgery, International Society of Nephrology, New York Academy of Sciences, and Society for Surgery of the Alimentary Tract
Disclosure: AMGEN Royalty Consulting; ARdelyx Ownership interest Board membership
Dale K Mueller, MD Clinical Associate Professor of Surgery, Section Chief, Department of Surgery, University of Illinois College of Medicine; Co-Medical Director, Thoracic Center of Excellence, Vice-Chair, Department of Cardiovascular Medicine and Surgery, OSF St Francis Medical Center; Director, Adult ECMO, Cardiovascular and Thoracic Surgeon, HeartCare Midwest, SC
Dale K Mueller, MD is a member of the following medical societies: American College of Chest Physicians, American College of Surgeons, American Medical Association, American Medical Writers Association, Chicago Medical Society, Illinois State Medical Society, and Society of Thoracic Surgeons
Disclosure: Nothing to disclose.
Sri R Navaratnam, MBBS, PhD, FRCPC Assistant Professor, Department of Internal Medicine, Section of Hematology/Oncology, University of Manitoba; Consulting Medical Oncologist, Department of Hematology/Oncology, Cancer Care Manitoba
Norvin Perez, MD Medical Director, Juneau Urgent and Family Care
Norvin Perez, MD is a member of the following medical societies: American College of Emergency Physicians and American Medical Association
Disclosure: Nothing to disclose.
Stephen P Peters, MD, PhD, FACP, FAAAAI, FCCP, FCPP Professor of Genomics and Personalized Medicine Research, Internal Medicine, and Pediatrics, Associate Director, Center for Genomics and Personalized Medicine Research, Director of Research, Section on Pulmonary, Critical Care, Allergy and Immunologic Diseases, Wake Forest University School of Medicine
Stephen P Peters, MD, PhD, FACP, FAAAAI, FCCP, FCPP is a member of the following medical societies: American Academy of Allergy Asthma and Immunology, American Association of Immunologists, American College of Chest Physicians, American College of Physicians, American Federation for Medical Research, American Thoracic Society, and Sigma Xi
Disclosure: See below for list of all activities None None
Daniel S Schwartz, MD, FACS Assistant Clinical Professor of Cardiothoracic Surgery, Mount Sinai School of Medicine; Chief of Thoracic Surgery, Huntington Hospital
Daniel S Schwartz, MD, FACS is a member of the following medical societies: American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, and Western Thoracic Surgical Association
Disclosure: Nothing to disclose.
Sat Sharma, MD, FRCPC Professor and Head, Division of Pulmonary Medicine, Department of Internal Medicine, University of Manitoba; Site Director, Respiratory Medicine, St Boniface General Hospital
Sat Sharma, MD, FRCPC is a member of the following medical societies: American Academy of Sleep Medicine, American College of Chest Physicians, American College of Physicians-American Society of Internal Medicine, American Thoracic Society, Canadian Medical Association, Royal College of Physicians and Surgeons of Canada, Royal Society of Medicine, Society of Critical Care Medicine, and World Medical Association
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Richard Thurer, MD B and Donald Carlin Professor of Thoracic Surgical Oncology, University of Miami, Leonard M Miller School of Medicine
Richard Thurer, MD is a member of the following medical societies: American Association for Thoracic Surgery, American College of Chest Physicians, American College of Surgeons, American Medical Association, American Thoracic Society, Florida Medical Association, Society of Surgical Oncology, and Society of Thoracic Surgeons
Disclosure: Nothing to disclose.
References
- Lillington GA. Management of solitary pulmonary nodules. Dis Mon. 1991 May. 37(5):271-318. [Medline].
- Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007 Sep. 132(3 Suppl):94S-107S. [Medline].
- Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007 Sep. 132(3 Suppl):108S-130S. [Medline].
- Cardinale L, Ardissone F, Novello S, Busso M, Solitro F, Longo M, et al. The pulmonary nodule: clinical and radiological characteristics affecting a diagnosis of malignancy. Radiol Med. 2009 Sep. 114(6):871-89. [Medline].
- Swensen SJ, Viggiano RW, Midthun DE, Müller NL, Sherrick A, Yamashita K, et al. Lung nodule enhancement at CT: multicenter study. Radiology. 2000 Jan. 214(1):73-80. [Medline].
- Midthun DE, Swensen SJ, Jett JR, Hartman TE. Evaluation of nodules detected by screening for lung cancer with low dose spiral computed tomography. Lung Cancer. Aug 2003;41 (suppl 2):S40.:
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003 Jun 19. 348(25):2535-42. [Medline].
- Xu DM, van der Zaag-Loonen HJ, Oudkerk M, Wang Y, Vliegenthart R, Scholten ET, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology. 2009 Jan. 250(1):264-72. [Medline].
- Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology. 2009 Dec. 253(3):606-22. [Medline].
- Ahn MI, Gleeson TG, Chan IH, McWilliams AM, Macdonald SL, Lam S, et al. Perifissural nodules seen at CT screening for lung cancer. Radiology. 2010 Mar. 254(3):949-56. [Medline].
- Turan O, Ozdogan O, Gurel D, Onen A, Kargi A, Sevinc C. GROWTH OF A SOLITARY PULMONARY NODULE AFTER 6 YEARS DIAGNOSED AS ONCOCYTIC CARCINOID TUMOUR WITH A HIGH 18-FLUORODEOXYGLUCOSE (FDG) UPTAKE IN POSITRON EMISSION TOMOGRAPHY-COMPUTED TOMOGRAPHY (PET-CT). Clin Respir J. 2011 Nov 30. [Medline].
- Behzadi A, Ung Y, Lowe V, Deschamps C. The role of positron emission tomography in the management of non-small cell lung cancer. Can J Surg. 2009 Jun. 52(3):235-42. [Medline]. [Full Text].
- Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001 Feb 21. 285(7):914-24. [Medline].
- Goldsmith SJ, Kostakoglu L. Role of nuclear medicine in the evaluation of the solitary pulmonary nodule. Semin Ultrasound CT MR. 2000 Apr. 21(2):129-38. [Medline].
- Yi CA, Lee KS, Kim BT, Choi JY, Kwon OJ, Kim H, et al. Tissue characterization of solitary pulmonary nodule: comparative study between helical dynamic CT and integrated PET/CT. J Nucl Med. 2006 Mar. 47(3):443-50. [Medline].
- Tsunezuka Y, Shimizu Y, Tanaka N, Takayanagi T, Kawano M. Positron emission tomography in relation to Noguchi's classification for diagnosis of peripheral non-small-cell lung cancer 2 cm or less in size. World J Surg. 2007 Feb. 31(2):314-7. [Medline].
- Naalsund A, Maublant J. The solitary pulmonary nodule--is it malignant or benign? Diagnostic performance of Tc-depreotide SPECT. Respiration. 2006. 73(5):634-41. [Medline].
- van 't Westeinde SC, Horeweg N, Vernhout RM, Groen HJ, Lammers JW, Weenink C, et al. The role of conventional bronchoscopy in the work-up of suspicious CT screen detected pulmonary nodules. Chest. 2012 Feb 2. [Medline].
- Herth FJ, Eberhardt R, Becker HD, Ernst A. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: a prospective trial. Chest. 2006 Jan. 129(1):147-50. [Medline].
- Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002 Oct. 20(4):972-4. [Medline].
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-Analysis of Guided Bronchoscopy for the Evaluation of the Pulmonary Nodule. Chest. 2011 Oct 6. [Medline].
- Lacasse Y, Wong E, Guyatt GH, Cook DJ. Transthoracic needle aspiration biopsy for the diagnosis of localised pulmonary lesions: a meta-analysis. Thorax. 1999 Oct. 54(10):884-93. [Medline]. [Full Text].
- Chung T. Fine needle aspiration of the solitary pulmonary nodule. Semin Thorac Cardiovasc Surg. 2002 Jul. 14(3):275-80. [Medline].
- Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest. 2003 Jan. 123(1 Suppl):89S-96S. [Medline].
- MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005 Nov. 237(2):395-400. [Medline].
- Gurney JW. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part I. Theory. Radiology. 1993 Feb. 186(2):405-13. [Medline].
- Watanabe A, Koyanagi T, Obama T, Ohsawa H, Mawatari T, Takahashi N, et al. Assessment of node dissection for clinical stage I primary lung cancer by VATS. Eur J Cardiothorac Surg. 2005 May. 27(5):745-52. [Medline].
- Shennib H. Intraoperative localization techniques for pulmonary nodules. Ann Thorac Surg. 1993 Sep. 56(3):745-8. [Medline].
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995 Sep. 60(3):615-22; discussion 622-3. [Medline].
- Cummings SR, Lillington GA, Richard RJ. Estimating the probability of malignancy in solitary pulmonary nodules. A Bayesian approach. Am Rev Respir Dis. 1986 Sep. 134(3):449-52. [Medline].
- Dholakia S, Rappaport DC. The solitary pulmonary nodule. Is it malignant or benign?. Postgrad Med. 1996 Feb. 99(2):246-50. [Medline].
- Luckraz H, Amer K, Thomas L, Gibbs A, Butchart EG. Long-term outcome of bronchoscopically resected endobronchial typical carcinoid tumors. J Thorac Cardiovasc Surg. 2006 Jul. 132(1):113-5. [Medline].
- Gildea TR, Mazzone PJ, Karnak D, Meziane M, Mehta AC. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med. 2006 Nov 1. 174(9):982-9. [Medline]. [Full Text].
- Kaiser LR, Shrager JB. Video-assisted thoracic surgery: the current state of the art. AJR Am J Roentgenol. 1995 Nov. 165(5):1111-7. [Medline].
- Landreneau RJ, Mack MJ, Hazelrigg SR, Dowling RD, Acuff TE, Magee MJ, et al. Video-assisted thoracic surgery: basic technical concepts and intercostal approach strategies. Ann Thorac Surg. 1992 Oct. 54(4):800-7. [Medline].
- Landreneau RJ, Hazelrigg SR, Mack MJ, Dowling RD, Burke D, Gavlick J, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993 Dec. 56(6):1285-9. [Medline].
- Travis WD, Rush W, Flieder DB, Falk R, Fleming MV, Gal AA, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998 Aug. 22(8):934-44. [Medline].
- Kuyumcu S, Adalet I, Sanli Y, Turkmen C, Ozkan ZG, Yilmazbayhan D. Somatostatin receptor scintigraphy with (111)In-octreotide in pulmonary carcinoid tumours correlated with pathological and (18)FDG PET/CT findings. Ann Nucl Med. 2012 Jul 17. [Medline].
- Gelbman BD, Cham MD, Kim W, Libby DM, Smith JP, Port JL, et al. Radiographic and Clinical Characterization of False Negative Results from CT-Guided Needle Biopsies of Lung Nodules. J Thorac Oncol. 2012 May. 7(5):815-20. [Medline].
Right upper lobe nodule shows peripheral calcification and high Hounsfield unit enhancement, suggesting that the lesion is a calcified, benign pulmonary nodule.
A 1.5-cm coin lesion in the left upper lobe in a patient with prior colonic carcinoma. Transthoracic needle biopsy findings confirmed this to be a metastatic deposit.
Mediastinal windows of the patient in the previous image
Right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
Close-up view of a right lower lobe nodule demonstrating central calcification. The most likely diagnosis is histoplasmosis.
Left upper lobe cavitating solitary nodule eventually identified as active pulmonary tuberculosis from percutaneous needle biopsy findings.
A left upper lobe nodule with central lucency and poorly circumscribed margins was diagnosed as actinomycosis based on needle biopsy findings.
Computed tomography (CT) scan of the patient in the previous image. After needle biopsy, the presence of classic sulfur granules confirmed a diagnosis of actinomycosis.
A right lower lobe solitary pulmonary nodule that was later identified as a hamartoma.
Wedge-shaped peripheral (pleural based) density observed secondary to pulmonary infarction (pulmonary embolism). This is termed the Hampton hump.
Left upper lobe 1.5-cm nodule shows negative computed tomography (CT) scan numbers, suggesting fat in the lesion consistent with hamartoma.
A left upper lobe solitary pulmonary nodule. The differential diagnosis in such cases is large, but computed tomography (CT) scan findings help to narrow the differentials and establish the diagnosis.
Cavitating right lower lobe nodule later confirmed to be primary pulmonary lymphoma. Calcium deposits may also be present in the lesion.
This left lower lobe carcinoid tumor was quite bloody after a percutaneous needle biopsy was performed.
Lateral radiograph of the patient in the previous image.
Computed tomography (CT) scan of a patient with a left lower lobe carcinoid tumor shows a well-circumscribed lesion.
A popcorn calcification in the left lung nodule indicates a benign lesion or hamartoma. No further tests or observations were needed for this patient.
A 1.5-cm right upper lobe nodule on a computed tomography (CT) scan was determined to be a benign, fibrous lesion on needle biopsy. A follow-up at 2 years showed no change in the size of this lesion.
The parenchymal lesion in this computed tomography (CT) scan demonstrates low attenuation within the lesion, indicating the presence of fat. Fat density is observed only in hamartoma and lipoid pneumonia. The likely diagnosis is hamartoma
This patient has a low risk for malignancy of the right upper lobe nodule. Therefore, continued observation with repeat chest radiographs to establish a growth pattern is the best treatment option.
Source...
